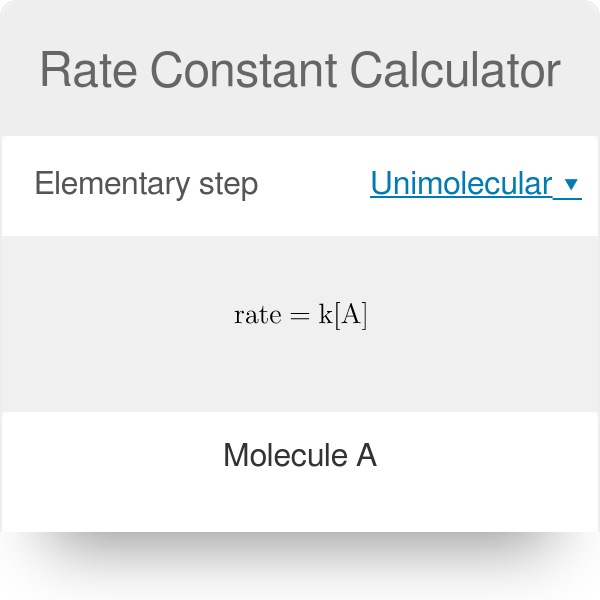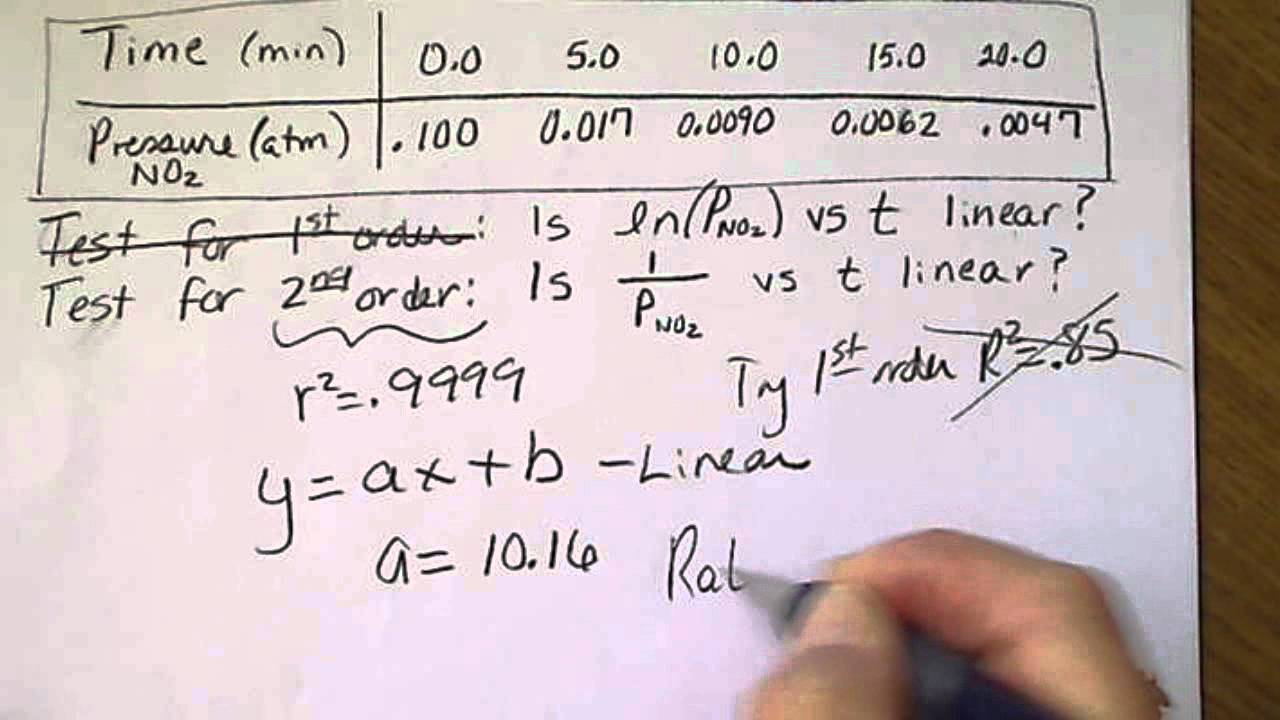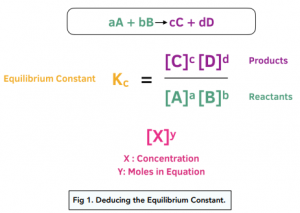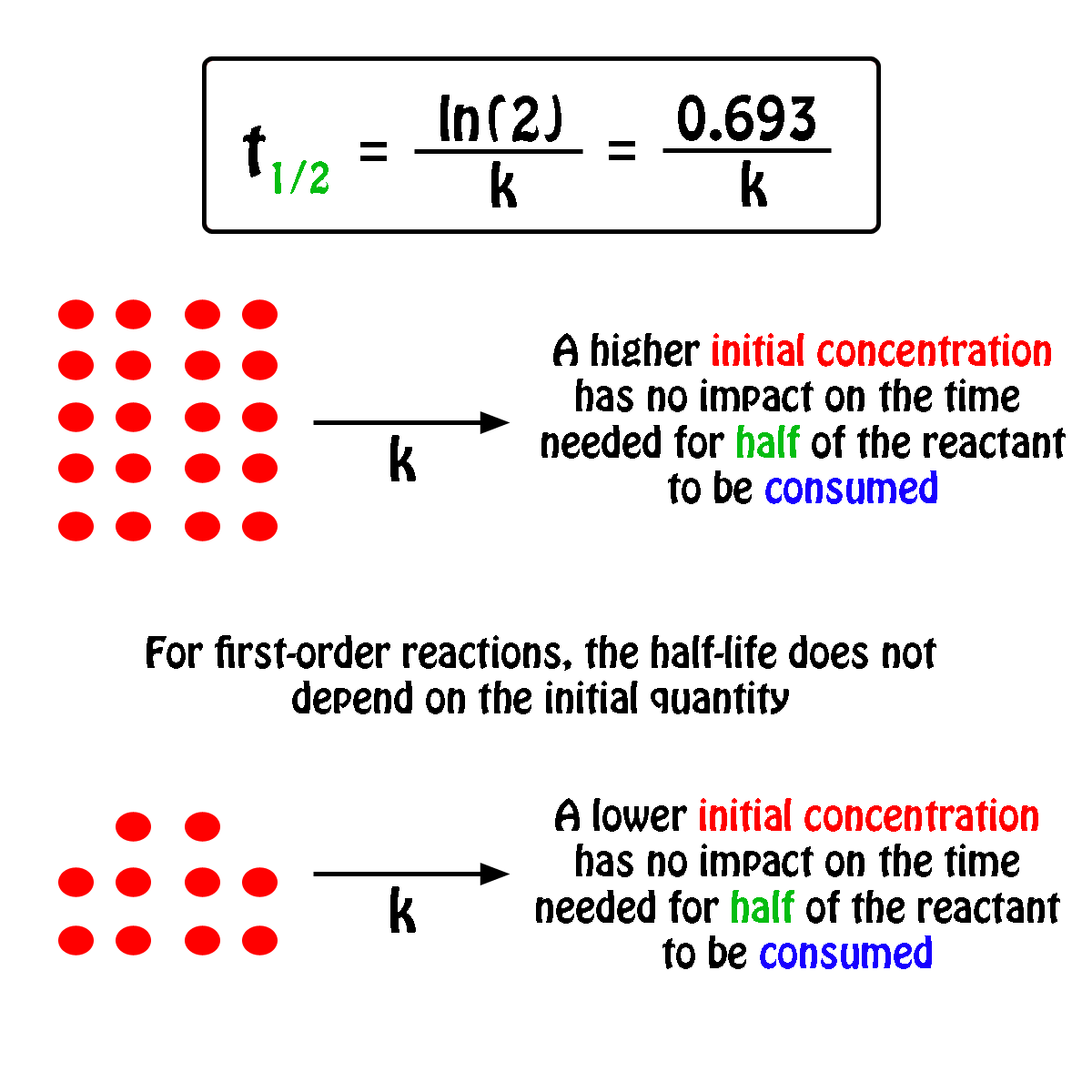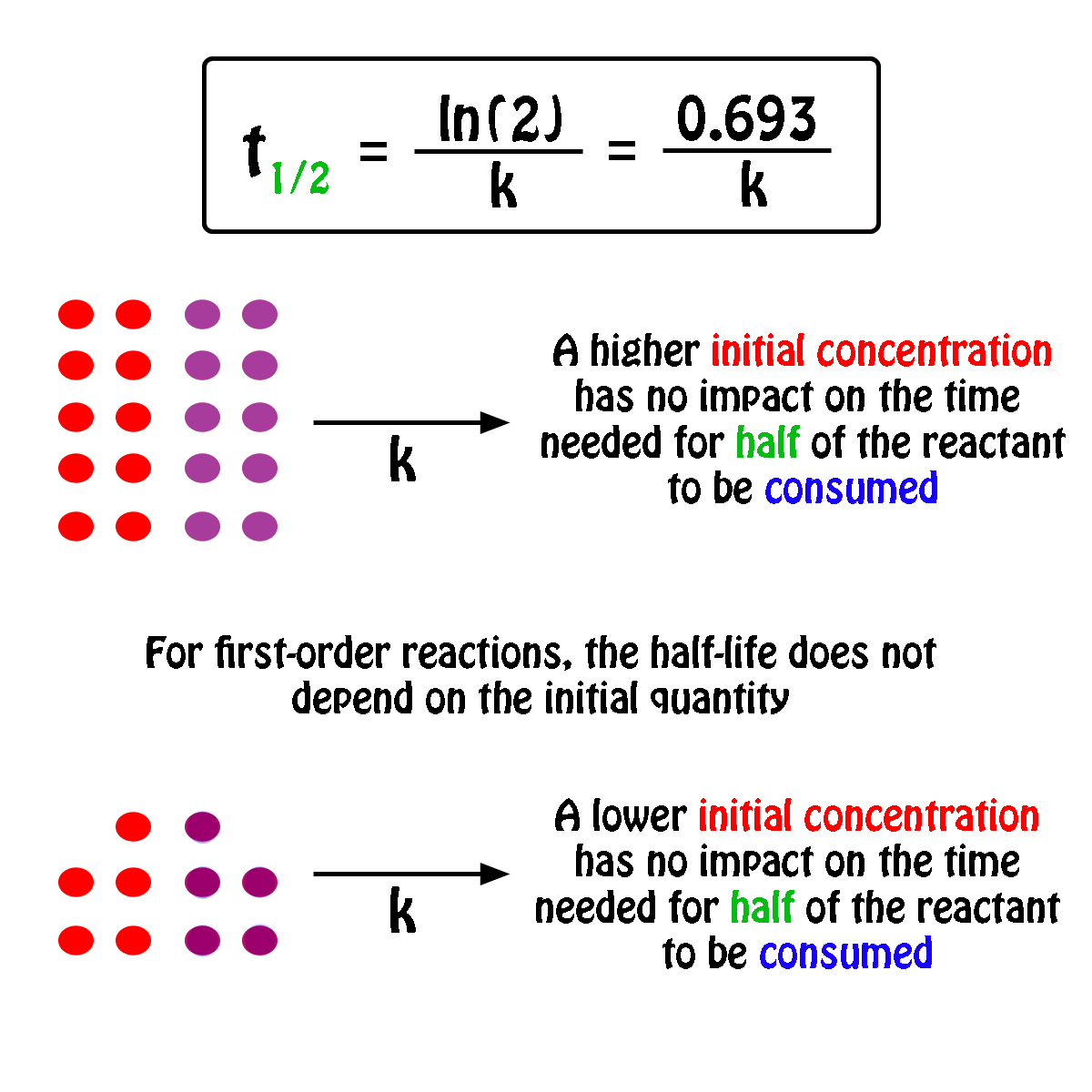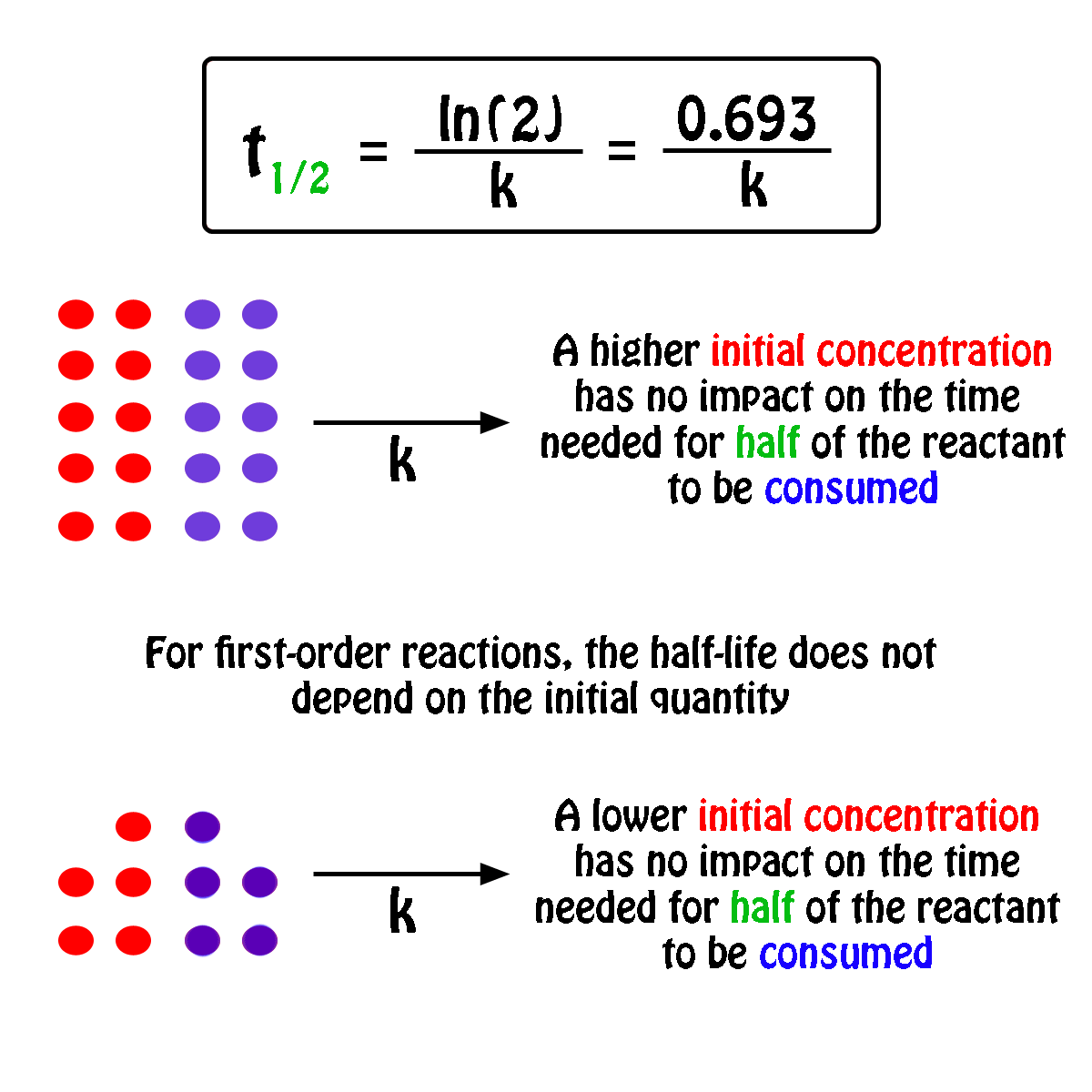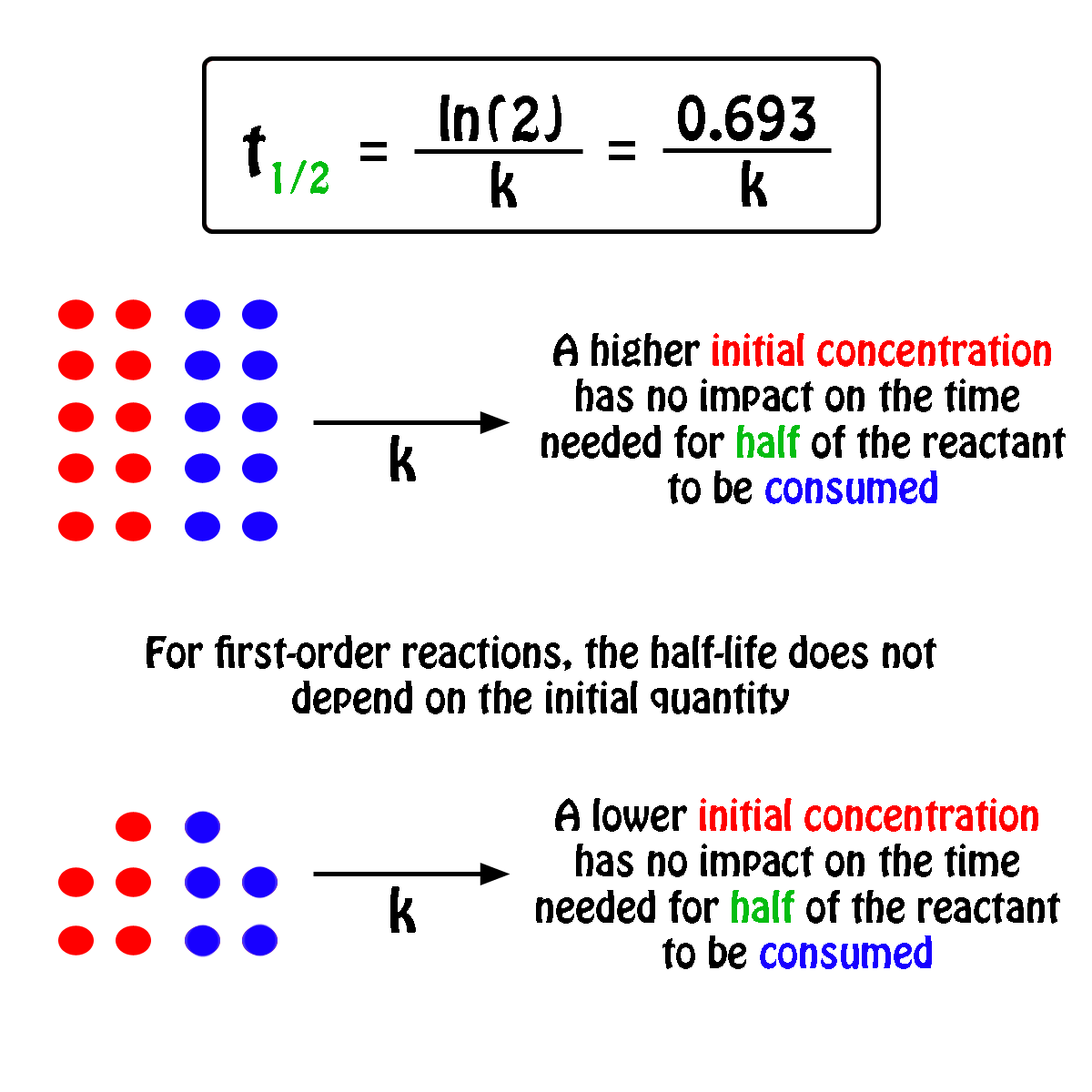
What is the half-life of a first-order reaction with a rate constant of 7.80xx10^-4 s^(-1)? | Socratic

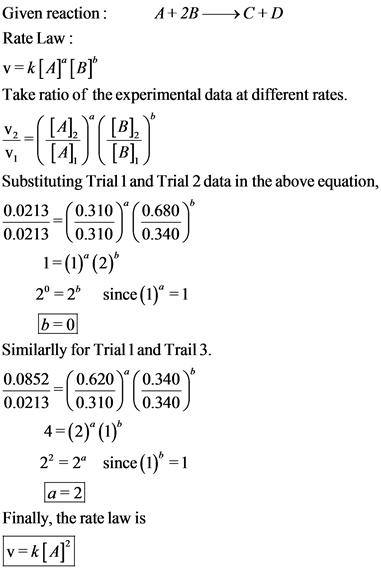
SOLVED: Ok, so I understand the process of doing this, but I'm having trouble inputting it into my calculator. The reaction CH3CHO(g) â†' CH4(g) + CO(g) has a rate constant of 0.213

Chemical Kinetics: The Integrated Rate Law- Calculating the Final Conc. for a Second Order Reaction - YouTube

The rate constant is given by the equation k = P.Ze^-E_a/RT . Which factor should register a decrease for the reaction to proceed more rapidly:

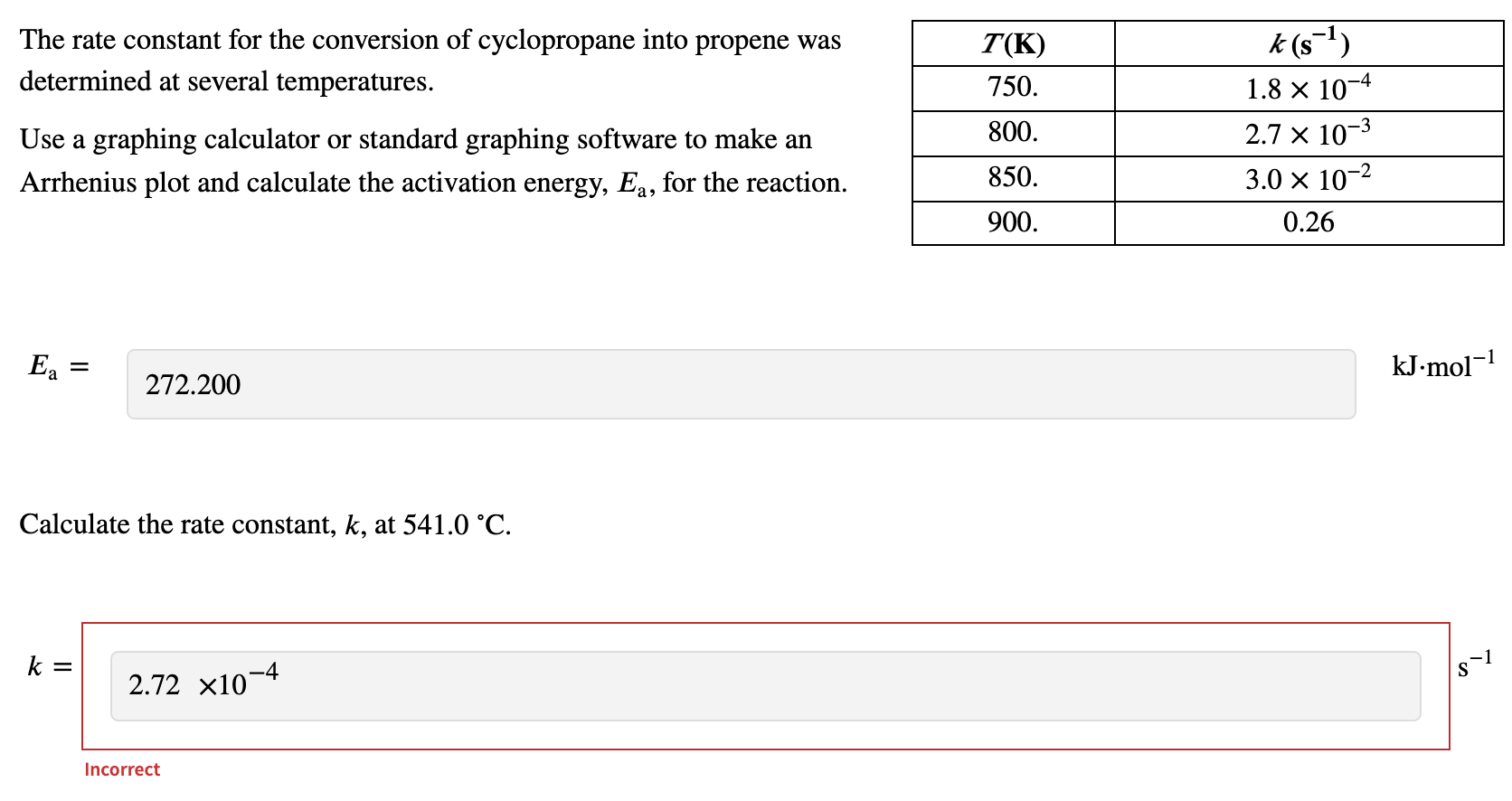
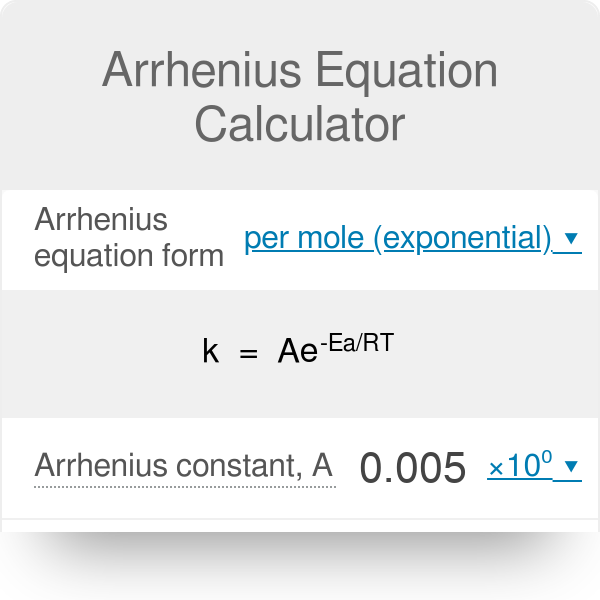
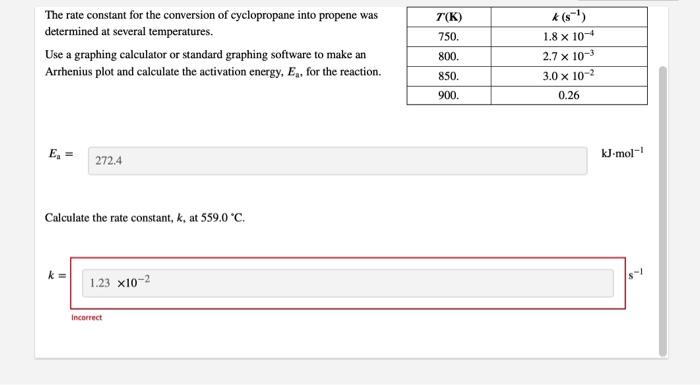
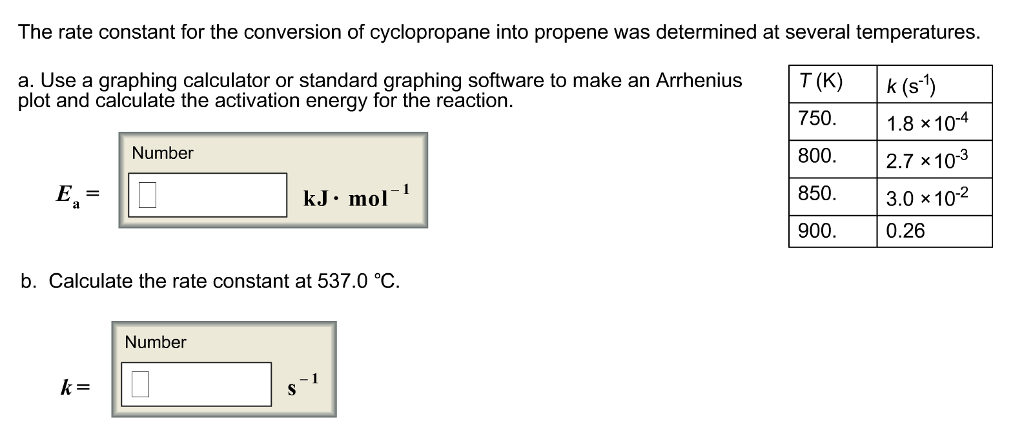
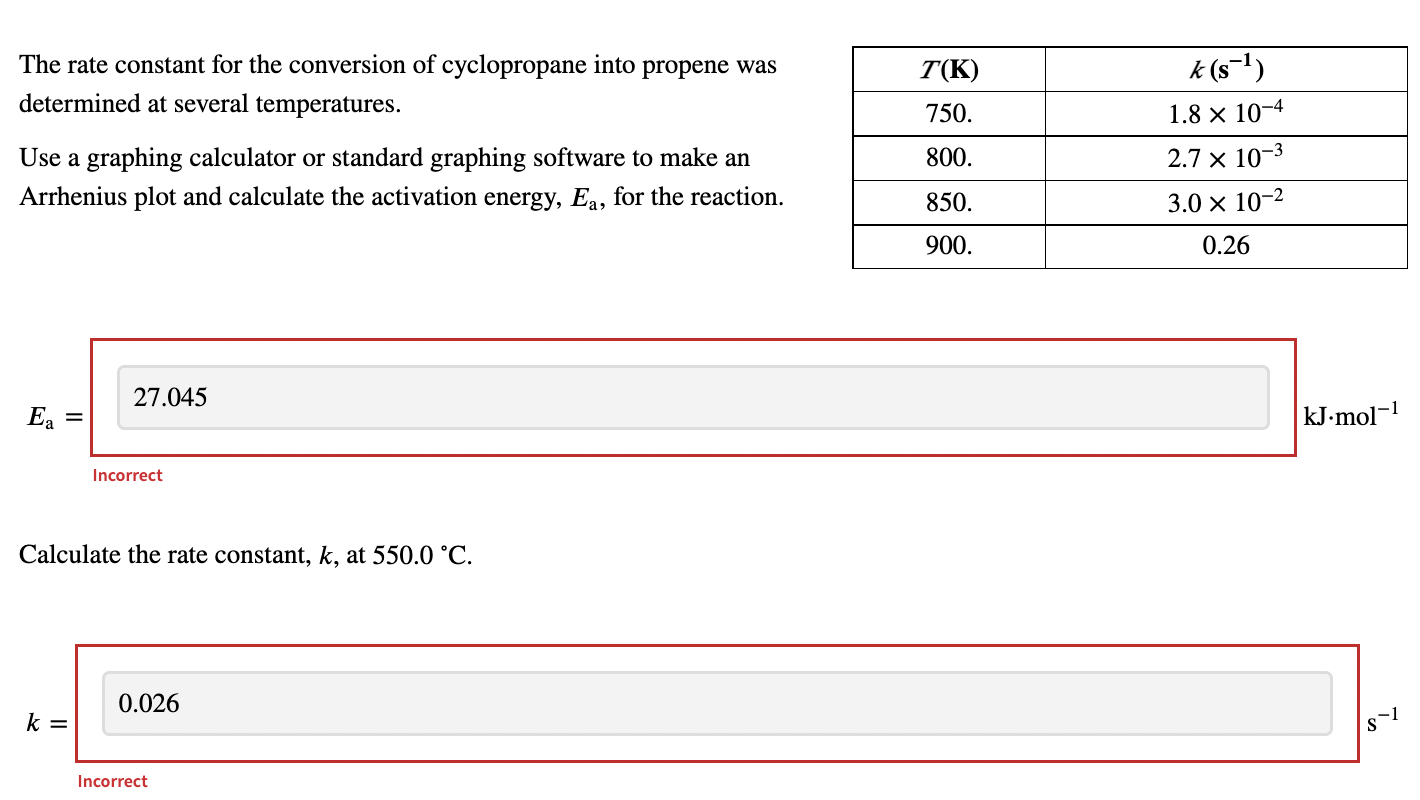
SOLVED: The rate constant for the conversion of cyclopropane into propene was determined at several temperatures. T (K) k (s^-1) 750 1.8x10^-4 800 2.7x10^-3 850 3.0x10^-2 900 0.26 Use a graphing calculator
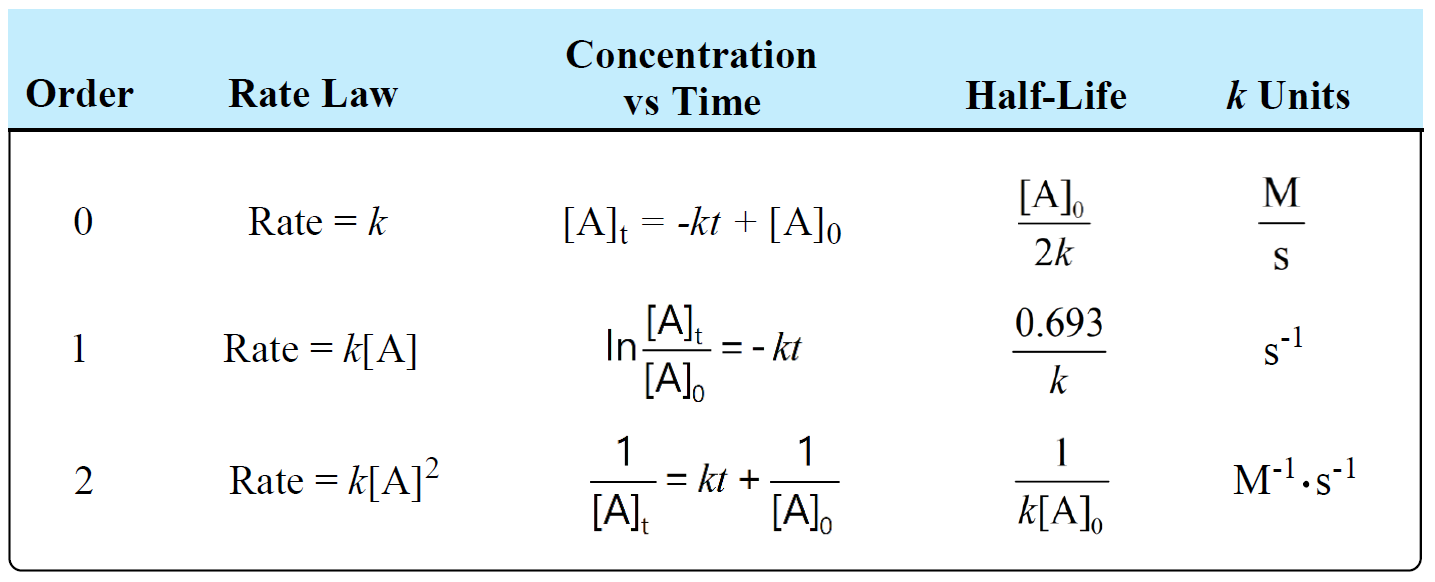
Concentration–Time Relationships: Integrated Rate Laws – Introductory Chemistry – 1st Canadian Edition